Physical properties
Besides its reducing efficiency, knowledge of its physical properties is also very important for every user of a reducing agent. And what physical properties are characteristic for Synhydrid®?
Besides its reducing efficiency, knowledge of its physical properties is also very important for every user of a reducing agent. And what physical properties are characteristic for Synhydrid®?
Appearance
At ambient temperature Synhydrid® is clear colourless liquid without turbidity.
Concentration of active ingredient
Synhydrid® is 70 % wt. toluene solution of sodium dihydridobis(2-methoxyethoxo) aluminate (SDMA). The concentration of SDMA is measured via determination of the hydride bonded hydrogen atoms content (active hydrogen). The most suitable method for active hydrogen determination is gas-volumetry based on measurement of generated hydrogen volume after water and hydrochloric acid addition to Synhydrid® sample. For each Synhydrid® batch we guarantee concentration between 70,5 and 71,5 % wt. of SDMA measured by volumetric method. For special cases we also offer Synhydrid 60 – the toluene solution of SDMA with SDMA concentration 60 % wt. (concentration range from 60,5 to 61,5 % wt.)
Solubility
Synhydrid® is soluble in ethers and aromatic hydrocarbons. In some ethers and aromatic hydrocarbons the solubility is practically unlimited. Both these kinds of solvents can be used in industrial scale reductions as the reaction media. The applicable solvents from the group of aromatic hydrocarbons are toluene and xylenes, from the group of ethers e.g. tetrahydrofuran, diethyl ether, methyl tert-butyl ether, anisole and monoglyme.
Stability in contact with the air
Synhydrid® exothermally reacts with air moisture but does not self-ignite as pyrophoric lithium aluminium hydride.
Synhydrid® in contact with the air moisture hydrolyzes and decomposes which leads to decrease of active hydrogen content. This hydride content decrease depends on the temperature and relative humidity of the air, surface area in contact with air, Synhydrid® volume and the time of exposure in the air.
In the beginning of hydrolysis in moist air, Synhydrid® does not become turbid in contrast to solution of lithium aluminium hydride because in first step soluble sodium alkoxyaluminate is formed.
In practice it means that for example in laboratory Synhydrid® can be poured quickly to inertized reaction vessel or dropping funnel without any risk of ignition or significant active hydrogen loss.
Thermal stability
Active constituent of Synhydrid® – SDMA – is thermally stable up to 170°C. Decomposition takes place above 170°C up to 214°C very slowly. Above this limit decomposition runs very quickly and spontaneously.
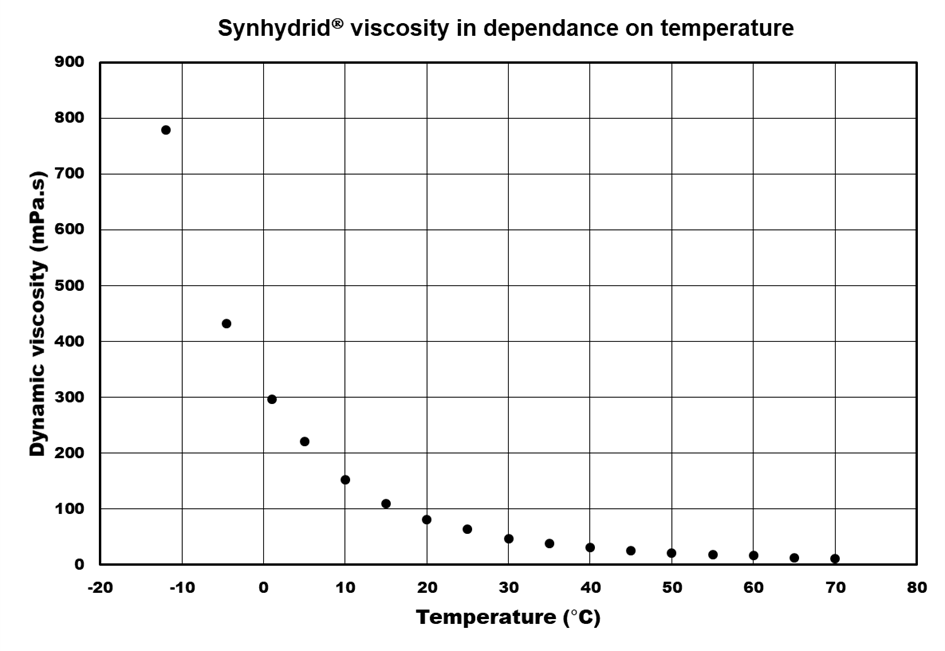
Viscosity
Although the concentration of SDMA in Synhydrid® 70 % wt. is quite high, the dynamic viscosity coefficient of such solution at 20°C lies around 80 mPa.s which is relatively low value. With decreasing temperature Synhydrid® viscosity increases but not extremely sharply. The viscosity of Synhydrid® at -10°C is around 700 mPa.s. From practical point of view it means that Synhydrid® can be easily pumped to reaction vessel at relatively wide range of temperatures.